The Čiháková research laboratory is an immunology laboratory dedicated to the investigation of autoimmune diseases. Our most active research is focused on myocarditis and dilated cardiomyopathy. We expanded our interest in inflammatory heart diseases to include the study of immune mechanisms driving pericarditis and myocardial infarction. In addition, we are interested in the pathogenesis of a broad range of autoimmune diseases such as, Sjogren’s syndrome, congenital complete heart block, and APECED (autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy). Through several collaborative projects we also investigate rheumatoid arthritis and the immune components of schizophrenia.
My laboratory examines 2 main areas related to COVID-19:
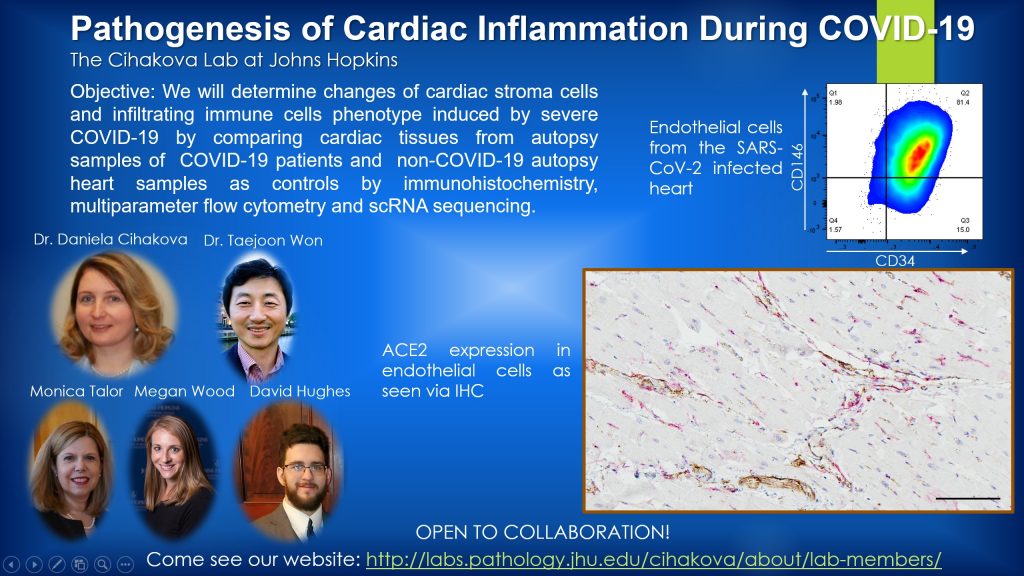
1.The global pandemic of coronavirus disease-2019 (COVID-19) caused by infection by a novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) affects mostly respiratory tract of. However, hospitalized COVID-19 patients have also increased incidence of venous and arterial thromboembolic complications including acute pulmonary embolism, deep-vein thrombosis, ischemic stroke, myocardial infarction or systemic arterial embolism. It remains unclear, however, what the actual changes of the endothelial cells in COVID-19 are and whether these changes are induced by direct infection of endothelial cells by SARS-CoV-2 or indirectly by cytokines or hypoxia. We study endothelial cell changes in COVID-19 patients’ lungs, hearts, and kidneys in collaboration with Dr. Jody Hooper and Dr. Andrew S. Pekosz. Using autopsy specimens of patients that died from COVID-19, we found that endothelial cells of COVID-19 lungs were in a dysfunctional state that promote coagulation in fatal COVID-19. We will farther examine how these changes on the endothelial cells are induced utilizing the COVID-19 autopsy samples and mice models infected with SASR-CoV-2 virus.
2.Myocarditis (inflammation of the heart) has been identified as a serious complication of SARS-CoV-2 infection. We will employ three approaches to understand how the SARS-CoV-2 is attacking the heart. First, we will use mouse models to identify COVID-19 receptors, such as ACE2 and DS-SIGN expression by cardiomyocytes, cardiac fibroblasts, endothelial cells, and other cardiac stroma cells. Second, in collaboration with Nisha Gilotra (Department of Cardiology, JHU), we will compare the immune profile of patients with COVID-19 myocarditis and also patients with so called long Covid and cardiac complications and those without the heart involvement. Third, we will examine antibodies against cardiac tissue to explore a hypothesis that there is an autoimmune component in the COVID-19 associated myocarditis.