Research Description
- Antigen Presentation and Recognition by CD4+ T cells
CD4+T cells of the immune system recognize peptide fragments of self or foreign antigens that are loaded on MHC-II molecules. What biochemical and molecular factors determine which peptides of a given antigen –“immunodominant epitopes” — are recognized? Understanding these factors is critically important, as alterations in the quality and quantity of MHC-II antigen presentation has major implications for the induction of immunologic memory, tolerance, and disease. In fact, it has now become clear that MHC-II antigen processing and presentation plays a major role in the pathophysiology of several autoimmune disorders including MS, infectious diseases such as HIV, and several cancers, including lung cancer.
Our lab has been at the forefront of defining the nature of MHC-II antigen processing and how this affects CD4+ T cell recognition and effector function. Among some of our past work, we have shown that having a peptide is essential for the MHC-II structure, determined the number and nature of peptide:MHC contacts to induce activation or hyporesponsiveness (anergy) in CD4T cells, and defined how a protein chaperone HLA-DM allows for selection of high-affinity peptides that can be stably presented to CD4+T cells. Using the biochemical principles we elucidated from these early studies, we developed a reductionist cell-free MHC-II processing system that is able to identify immunodominant epitopes of a given protein antigen. This system has identified critical epitopes in proteins implicated in autoimmunity as well as protein antigens from influenza, malaria, and HIV-1. During the past few years, we have been investigating the mechanism of another molecular chaperone, HLA-DO, in MHC-II antigen processing and its role in T cell selection and autoimmunity. Finally, we have recently began a foray into human immunology to investigate the purpose of inducible MHC-II expression on CD4+T cells, a cell that normally is thought to exclusively respond to, but not present its own, peptides. With these studies, we aim to precisely define the critical molecular determinants of peptide antigen presentation on MHC-II molecules in both the murine and human immune systems. Our goal is ultimately to understand how alterations in peptide presentation and immunodominance can lead to human immune disease, and how to better develop vaccines for infections and cancer that promote T cell recognition of the correct antigenic epitopes.
- Antigen processing is what we love
A simplified version of intracellular antigen processing pathway for MHC II for identification of immunodominant (immunogenic) epitopes.

Cell free antigen processing System finds immunodominant epitope
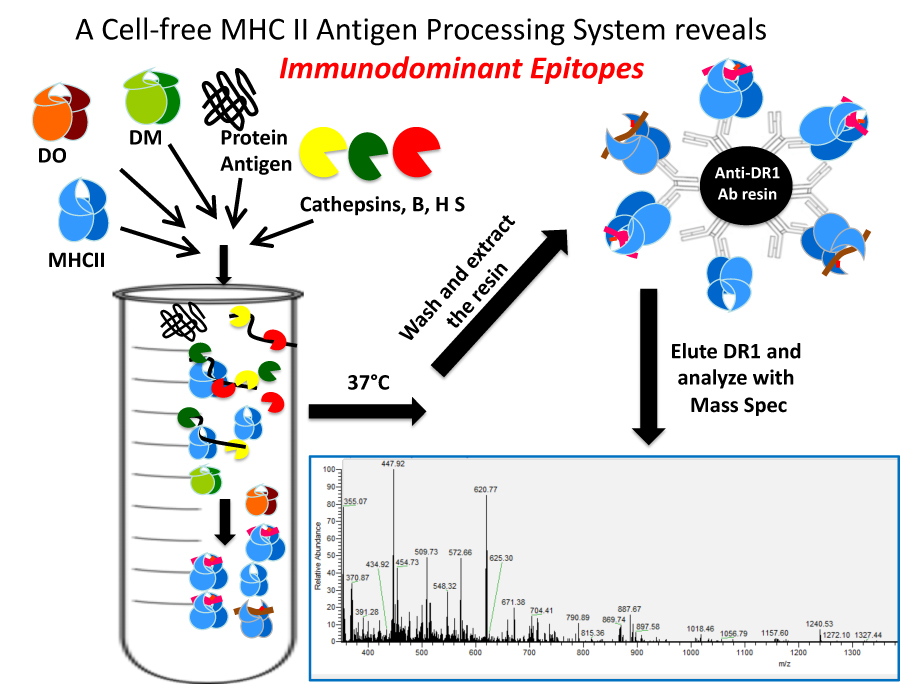

Current projects include:
- Understanding the biology of accessory molecule HLA-DO (H2-O in mice)
- Impacts of DO on T cell selection and its influence on the production of autoreactive T cells,
- Investigating other effects of DO of HLA-DO in immune system.
- Phenotypically defining markers of long-term memory CD4+T cells in mice.
- Understanding the role of MHC-II molecules on human CD4+ T cells, especially whether these molecules allow for the presentation of peptides from viruses like HIV that infect such cells.
Please email for further description on current and available projects!
DR1/Collagen immunodominant epitope Tetramer injected in vivo colocalizes with damaged collagen in CIA diseased mice





