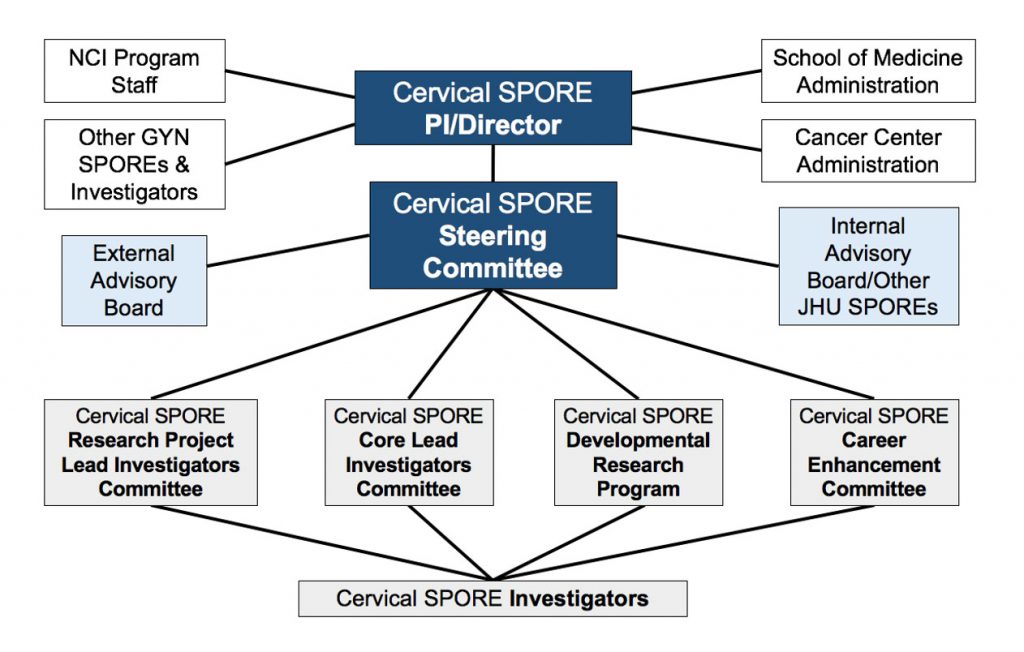
The Administration/Communication Core (Core A) functions as a resource for coordination, oversight, evaluation, and planning of the SPORE. Under the direction of Drs. Wu and Huh, the core coordinates activities of the review committees that oversee Program research activities and program development. This core will also prepare annual scientific reports and budgetary information that is essential for critical assessment and appropriation of resources.
Co-Investigators (basic and clinical) for each project will be responsible for directing the research outlined in their proposal. Selected members sit on the steering committee and attend meetings and seminars every month where the scientific progress and direction of the program is discussed. This monthly SPORE meeting has proven very valuable in the exchange of ideas. At least once per year, all Investigators and key personnel attend a meeting with external and internal advisors (letters of participation enclosed in Core A). Critical evaluation of the work in progress and future direction is discussed at this meeting. These advisory board meetings have successfully shaped nurtured and redirected underperforming projects. Each Co-Investigator is responsible for the preparation of progress reports and budgets for their projects to be presented in advance to the advisors that comprise the oversight committee. The Director of the SPORE program will also contact these advisors as needed to help guide individual projects through unforeseen problems and to continue the scientific and translational focus of the overall program. Finally, these individuals will also be available to advise and assist all the Co-Investigators and core directors as needed.
The core directors are responsible for the day-to-day oversight of their core’s activities. Selected members sit on the steering committee and attend seminars and review meetings as described above. They also submit progress reports and budgets annually. All key personnel are invited to the quarterly meetings to ensure maximal input and collaboration in all phases of the individual projects and core facilities. Further plans for SPORE Program project oversight and review (including critical clinical features) are presented in the Administration/Communication Core section. It is the responsibility of the Administration and Communication Core to facilitate the activities of the steering and review committees to fulfill these functions. Duties include scheduling and publicizing symposia, arranging quarterly internal review and annual external review meetings, planning meals and accommodations for external reviewers, selecting and integrating review committee and budgetary reports into annual reports for the Hopkins Research Administration and NCI. Administration of these activities is handled in Dr. Wu’s office with the help of his administrative assistant.
An Institutional Advisory Board consisting of senior investigators with experience related to various aspects of the proposed projects in the Program has been assembled by Dr. Wu. The advisory board has provided advice and direction for project Lead Investigators both individually and in scientific organizational seminars held during the initial funding period and for the development of the competitive renewal proposal. They will continue to be available as consultants and will be convened quarterly for review of the scientific progress of the SPORE and in annual meetings with the Program director and steering committee. Individual project investigators interact with specific advisory panel members more frequently on an informal basis for advice regarding their activities. A rotating panel of experts from outside of the Johns Hopkins Medical Institutions has also been recruited to serve on an External Advisory Panel. They hear presentations of progress reports and prospective pilot ideas. They provide candid advice to the Lead Investigators, Co-Lead Investigators and Core Directors and their feedback will be included in the annual report to the NCI. They are also available for ad hoc queries and advice throughout the year to individual Co-Investigators in their related fields. A complete list of the internal and external advisors, their credentials, and letters acknowledging the participation of all advisors in the SPORE program can be found in the Administration/Communication Core. The internal and external advisory panels will advise the steering committee for the SPORE project as described below.
The SPORE Steering Committee is the primary body responsible for research monitoring, evaluation and planning, quality assurance, fiscal responsibility, and decision-making for the entire SPORE program. The membership of the Steering Committee reflects the diversity of scientific expertise and administrative expertise needed for effective SPORE function. The committee will meet quarterly and as needed. External advisors may be asked to participate individually or as a group via phone conference at intervals between the yearly meetings. At each quarterly meeting, the progress of individual projects and cores will be evaluated. Problems or concerns are openly discussed and recommendations for improvement are made. Final approval of all scientific and research matters related to this program project rests on the steering committee by majority vote.
| Name | Position | Expertise |
|---|---|---|
| T.-C. Wu, M.D., Ph.D. | SPORE PI, Co-Leader of Project 4, Co-Director of Core A | HPV vaccines, immunology and pathology |
| Warner Huh, M.D. | SPORE PI, Clinical Research Director for SPORE, Co-Leader of Projects 2 and 3, Co-Director of Core A | Clinical trials, gynecologic oncology and pathology |
| Richard Roden, M.D. | Co-Leader of Projects 1 and 3 | HPV molecular virology and immunology |
| Robert Garcea, M.D. | Co-Leader of Project 1 | HPV molecular virology and immunology |
| Chien-Fu Hung, Ph.D. | Co-Leader of Project 2 | HPV vaccines, immunology, and pathology |
| Kimberly Lenvison, M.D. | Co-Leader of Projects 2 and 3 | Clinical trials, gynecology and obstetrics, cervical cancer |
| Stephanie Gaillard, M.D. | Co-Leader of Project 4 | Clinical trials, gynecologic oncology |
| Charles (Trey) Leath, III, M.D. | Co-Leader of Project 4 | Clinical trials, gynecologic oncology |
| Hao Wang, Ph.D. | Co-Director of Core B | Biostatistics and data management |
| Heba Mostafa, Ph.D. | Co-Director of Core B | Epidemiology, biostatistics and data management |
| Sejong Bae, Ph.D. | Co-Director of Core B | Biostatistics and data management |
| Raphael Viscidi, M.D. | Co-Director of Core C | Virology, Serology |
| Rebecca Arend, M.D., Ph.D. | Co-Leader of Project 2 and Co-Director of Core C | Cervical cancer pathology, cell therapy Gyn Oncology |
| Adrea Kahn, M.D. | Co-Director of Core C | Gynecologic pathology |
| Russell Vang, M.D. | Co-Director of Core C | Gynecologic pathology |
| Drew Pardoll, M.D., Ph.D. | Scientific Review Committee Chair | Molecular biology and immunology |
| Donald Buchsbaum, Ph.D. | Co-Leader of Career Enhancement Program | Immunology |