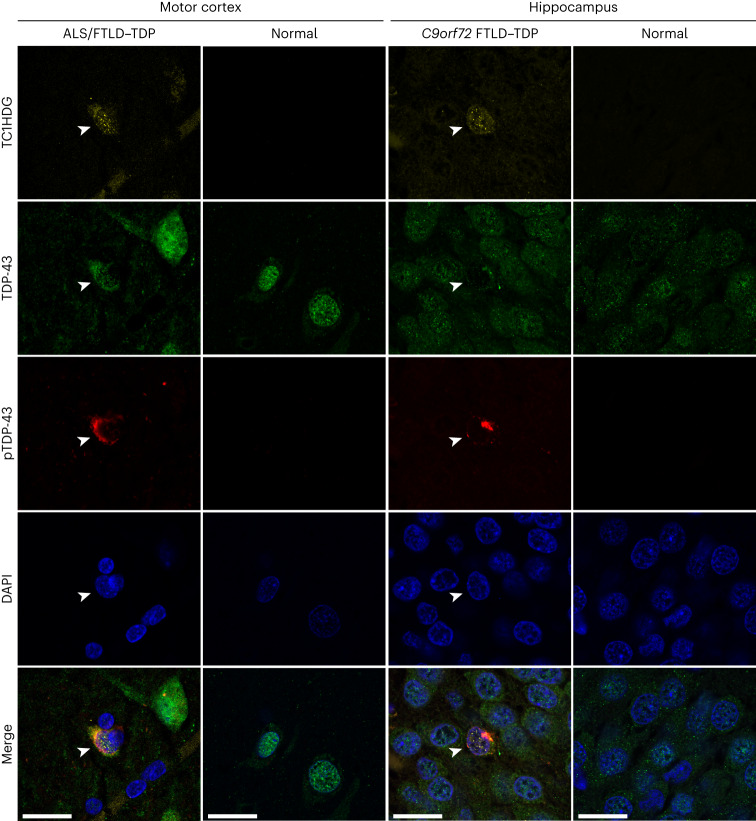
The Wong Laboratory is in the Division of Neuropathology at the Johns Hopkins University School of Medicine. The overarching themes focus on the biology and pathobiology of an RNA splicing factor termed TDP-43 (TAR DNA/RNA binding protein 43kDa) that regulates the inclusion of cryptic exons. The loss of TDP-43 underlies the pathogenic mechanism of several human age-related degenerative diseases, including Alzheimer’s Disease Related Dementia (ADRD), Amyotrophic Lateral Sclerosis (ALS), as well as Inclusion Body Myositis (IBM).

Current projects include the development of in vitro and in vivo models of TDP-43 loss-of-function in the nervous system and skeletal muscle to reveal its biology in a cell- or organ-specific manner.
Recent Related work(s):
Baghel, M.S., Burns, G.B. et al. bioRxiv (2024). DOI: 10.1101/2024.06.26.600814.
Current work involves the development of prognostic fluid biomarkers for these human diseases using in-frame cryptic exons.
Recent Related work(s):
Irwin, K.E. et al. Nature Med 30, 382-393 (2024). DOI: 10.1038/s41591-023-02788-5.
Recent Related work(s):
Donde, A. et al. Acta Neuropathol 138, 813-26 (2019). DOI: 10.1007/s00401-019-02042-8.
–TDP-43 repression of nonconserved cryptic exons is compromised in ALS-FTD
Ling, J.P. et al. TDP-43 repression of nonconserved cryptic exons is compromised in ALS-FTD. Science 349, 650-5 (2015). DOI:10.1126/science.aab0983
“In sporadic ALS (~97% of all cases) and sporadic FTD (~45% of all cases), TDP-43 clears from the nucleus and forms ubiquitinated, cytoplasmic inclusions, termed TDP-43 proteinopathy […] We have found that TDP-43 functions as a splicing repressor of nonconserved cryptic exons (fig. S8). A defect in this regulatory mechanism could be linked to TDP-43 proteinopathy in ALS-FTD.”
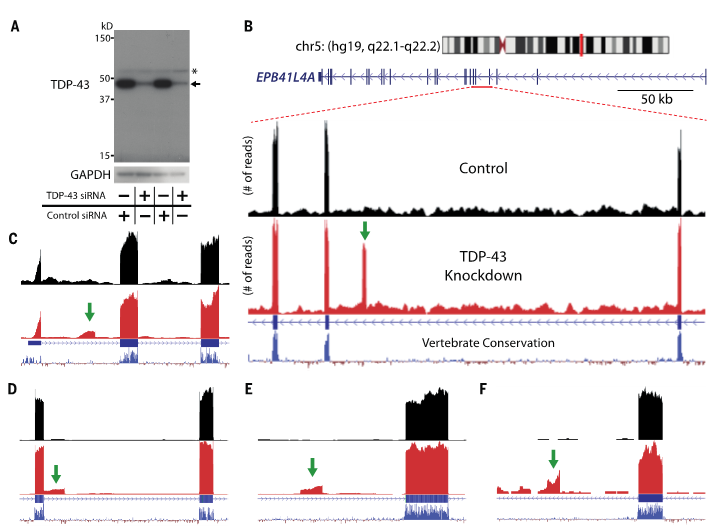
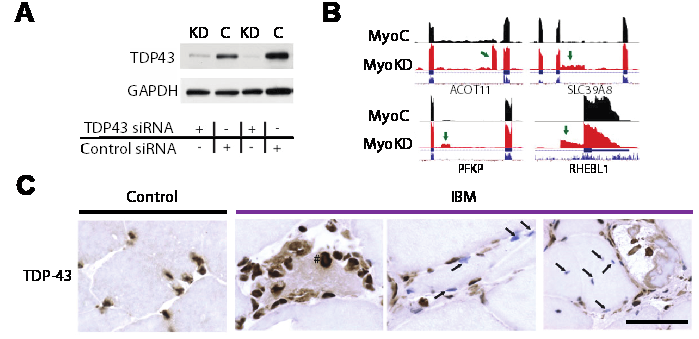
(A) TDP-43 protein levels are greatly reduced when HeLa cells are treated with TDP-43 siRNA (*, nonspecific band). (B) Visualization of the cryptic exon located in EPB41L4A. Zoom in of gene annotation demonstrates that the cryptic exon (green arrow) resides in a non-conserved region. Strand specific analysis also verifies the incorporation of cryptic exons on the transcribing strand (fig. S12). (C–F) IRF9 contains a transcriptional start site (C), KRT7 an exon extension (D), GPSM2 a standard cryptic exon (E), and INSR a polyadenylation site (F).
–Depletion of TDP-43 decreases fibril and plaque β-amyloid and exacerbates neurodegeneration in an AD mouse model.
LaClair, Katherine D et al. Depletion of TDP-43 decreases fibril and plaque β-amyloid and exacerbates neurodegeneration in an Alzheimer’s mouse model. Acta Neuropathol 132, 859-73 (2016). DOI:10.1007/s00401-016-1637-y
“Here, we show […] TDP-43 depletion in forebrain neurons of an AD mouse model exacerbates neurodegeneration, and correlates with increased prefibrillar oligomeric Aβ and decreased Aβ plaque burden. These findings support a role for nuclear depletion of TDP-43 in the pathogenesis of AD and provide strong rationale for developing novel therapeutics to alleviate the depletion of TDP-43 and functional antemortem biomarkers associated with its nuclear loss.”
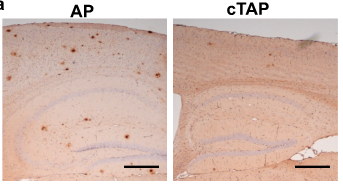
–Cryptic exon incorporation occurs in AD brain lacking TDP-43 inclusion but exhibiting nuclear clearance of TDP-43
Sun, M et al. Cryptic exon incorporation occurs in Alzheimer’s brain lacking TDP-43 inclusion but exhibiting nuclear clearance of TDP-43. Acta Neuropathol 133, 923-31 (2017). DOI:10.1007/s00401-017-1701-2
“We found that cryptic exon incorporation occurred in all AD cases exhibiting TDP-43 pathology. […] Importantly, cryptic exon incorporation could be detected in AD brains lacking TDP-43 inclusion but exhibiting nuclear clearance of TDP-43.”
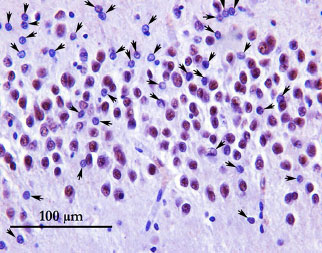
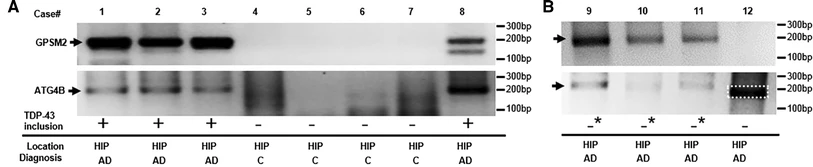
–Splicing repression is a major function of TDP-43 in motor neurons
Donde, Aneesh et al. Splicing repression is a major function of TDP-43 in motor neurons. Acta Neuropathol 138, 813-26 (2019). DOI:10.1007/s00401-019-02042-8
“AAV9-mediated delivery of [a] chimeric rescue repressor to mice lacking TDP-43 in motor neurons delayed the onset, slowed the progression of motor symptoms, and markedly extended their lifespan. In treated mice lacking TDP-43 in motor neurons, aberrant splicing was significantly decreased and accompanied by amelioration of axon degeneration and motor neuron loss. This AAV9 strategy allowed long-term expression of the chimeric repressor without any adverse effects.”
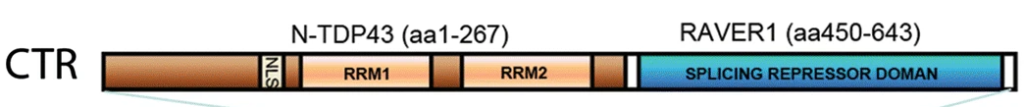
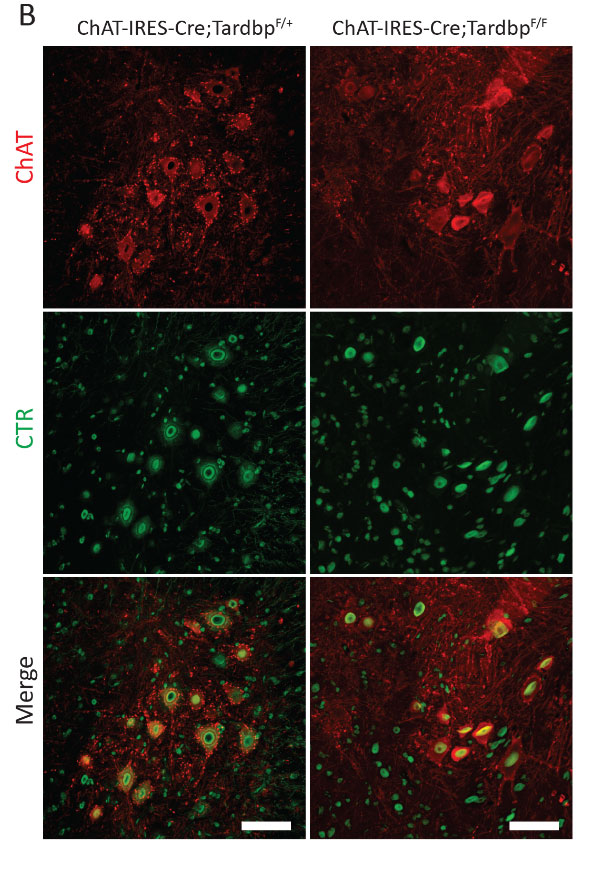
–Loss of TDP-43 function and rimmed vacuoles persist after T cell depletion in a xenograft model of sporadic inclusion body myositis
Britson, K.A. et al. Loss of TDP-43 function and rimmed vacuoles persist after T cell depletion in a xenograft model of sporadic inclusion body myositis. Sci Transl Med 14, eabi9196 (2022). DOI:10.1126/scitranslmed.abi9196
“IBM muscle biopsies display nuclear clearance and cytoplasmic aggregation of TDP-43 in muscle cells, a pathologic finding observed initially in neurodegenerative diseases, where nuclear loss of TDP-43 in neurons causes aberrant RNA splicing. Here, we show that loss of TDP-43–mediated splicing repression, as determined by inclusion of cryptic exons, occurs in skeletal muscle of subjects with IBM.“
–Cryptic HDGFL2 as a fluid biomarker reveals loss of TDP-43 splicing repression in pre-symptomatic ALS
Irwin, KE et al. A fluid biomarker reveals loss of TDP-43 splicing repression in presymptomatic ALS-FTD. Nature Med 30, 382-93 (2024). DOI:10.1038/s41591-023-02788-5
“Here we use a newly characterized monoclonal antibody specific to a TDP-43-dependent cryptic epitope (encoded by the cryptic exon found in HDGFL2) to show that loss of TDP-43 splicing repression occurs in ALS–FTD, including in presymptomatic C9orf72 mutation carriers. […] Our findings indicate that loss of TDP-43 cryptic splicing repression occurs early in disease progression, even presymptomatically, and that detection of the HDGFL2 cryptic neoepitope serves as a potential diagnostic biomarker for ALS[…]”