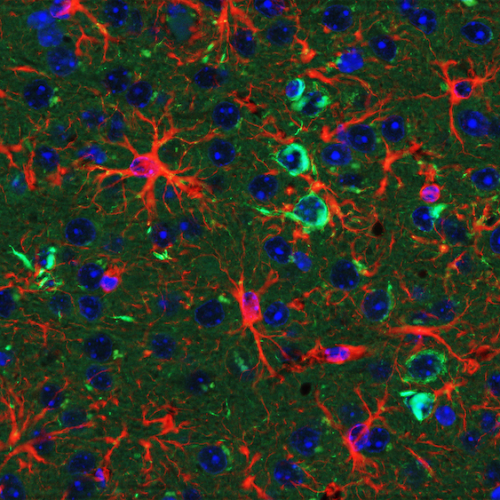
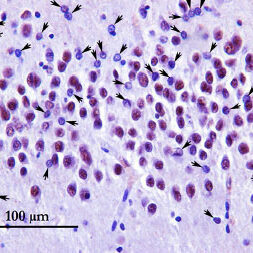
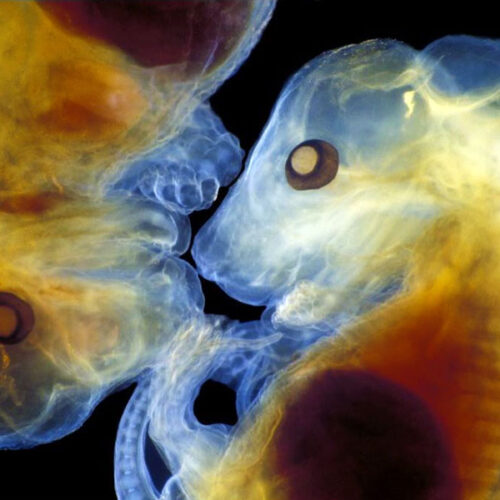
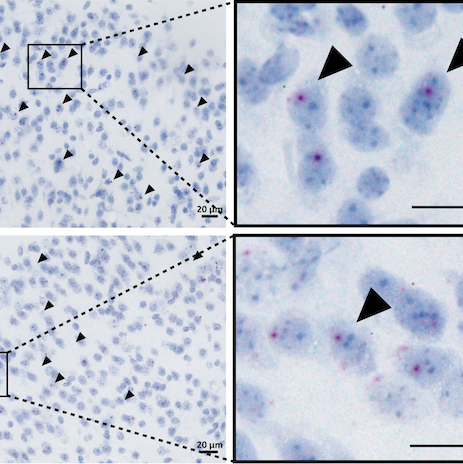
The Wong Laboratory is in the Division of Neuropathology at the Johns Hopkins University School of Medicine. The overarching themes focus on the biology and pathobiology of an RNA splicing factor termed TDP-43 (TAR DNA/RNA binding protein 43kDa) that regulate the inclusion of cryptic exons, the loss of which underlies the pathogenic mechanism of several human age-related degenerative diseases, including Alzheimer’s Disease Related Dementia (ADRD), Amyotrophic Lateral Sclerosis (ALS), as well as Inclusion Body Myositis (IBM).
Read more on our research page.