Immunoswitch: Dual Targeting Nanoparticles that Redirect the Anti-Tumor T Cell Response
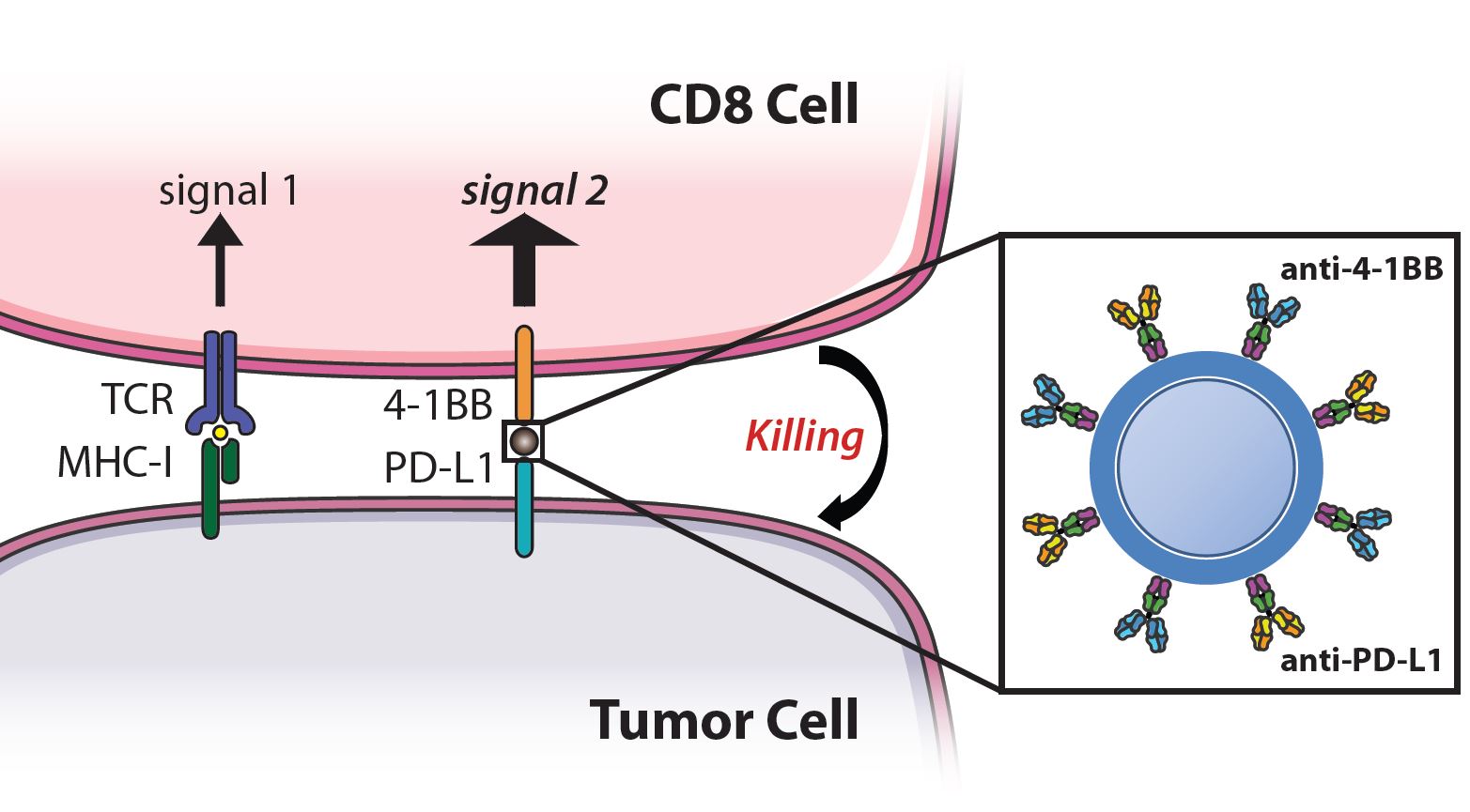 Recent advances in cancer immunotherapy have led to the advent of treatments that boost an anti-tumor response, such as checkpoint blockade and co-stimulatory molecules. However, these approaches are effective for only a subset of patients and target non-specific molecules, thus the large doses required result in off-target toxicities. We have developed a nanoparticle that synergizes checkpoint blockade and T cell costimulation on a single injectable therapeutic. Nanoparticles are conjugated with antibodies against 4-1BB, expressed by T cells, and anti-PD-L1, expressed by tumor cells and target these pathways while also physically linking the effector and target cell to increase efficacy. These dual targeting particles, termed immunoswitch particles, localize treatment to the tumor site and are effective at 10-100X lower doses than systemically injected drug and thus have the potential to reduce cost and off-target toxicities. Our lab is interested in further expanding upon this type of “signal-switching” particle by incorporating different combinations of signaling molecules and altering the material, shape, and size of the particle to improve biodistribution and interaction with T cells and tumor cells.
Recent advances in cancer immunotherapy have led to the advent of treatments that boost an anti-tumor response, such as checkpoint blockade and co-stimulatory molecules. However, these approaches are effective for only a subset of patients and target non-specific molecules, thus the large doses required result in off-target toxicities. We have developed a nanoparticle that synergizes checkpoint blockade and T cell costimulation on a single injectable therapeutic. Nanoparticles are conjugated with antibodies against 4-1BB, expressed by T cells, and anti-PD-L1, expressed by tumor cells and target these pathways while also physically linking the effector and target cell to increase efficacy. These dual targeting particles, termed immunoswitch particles, localize treatment to the tumor site and are effective at 10-100X lower doses than systemically injected drug and thus have the potential to reduce cost and off-target toxicities. Our lab is interested in further expanding upon this type of “signal-switching” particle by incorporating different combinations of signaling molecules and altering the material, shape, and size of the particle to improve biodistribution and interaction with T cells and tumor cells.
Biodegradable Artificial Antigen Presenting Cells
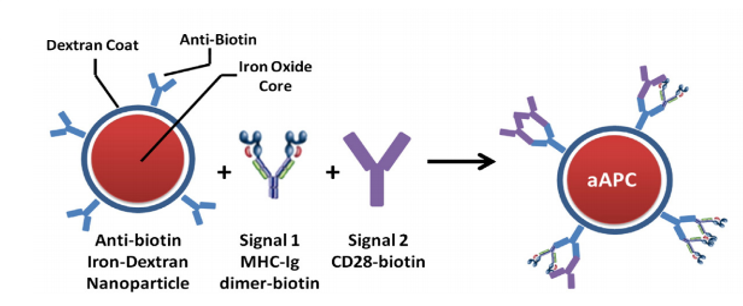 Artificial antigen presenting cells (aAPC) are cell-based or synthetic material-based three dimensional platforms capable of activating an anti-tumor T cell response. These platforms are decorated with the necessary signals required to activate T cells and can be used to stimulate T cells against a specific tumor antigen either ex vivo followed by adoptive cell transfer or directly in vivo. Synthetic aAPC have several advantages over cell-based aAPC because of their increased shelf-life, ability to engineer specific properties, and decreased cost. Our lab focuses on utilizing various engineering technologies to improve synthetic aAPC. We use superparamagnetic materials, biodegradable polymers, and variations in signaling molecules to develop next-generation aAPC.
Artificial antigen presenting cells (aAPC) are cell-based or synthetic material-based three dimensional platforms capable of activating an anti-tumor T cell response. These platforms are decorated with the necessary signals required to activate T cells and can be used to stimulate T cells against a specific tumor antigen either ex vivo followed by adoptive cell transfer or directly in vivo. Synthetic aAPC have several advantages over cell-based aAPC because of their increased shelf-life, ability to engineer specific properties, and decreased cost. Our lab focuses on utilizing various engineering technologies to improve synthetic aAPC. We use superparamagnetic materials, biodegradable polymers, and variations in signaling molecules to develop next-generation aAPC.
Mimicking APCs on the Nanoscale
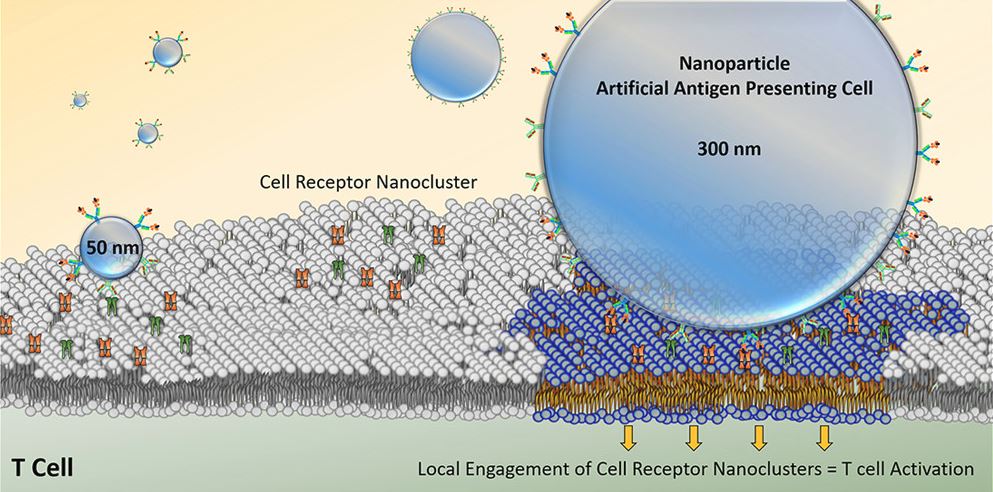 By controlling biomaterial properties, we can improve targeting of specific immune cells, mimic how the body activates immune cells, understand more about T cell biology, and increase the efficacy of T cell therapies. To mimic APCs we can create artificial APCs (aAPCs) by coating activating signals to the surface of magnetic nanoparticles. This magnetic platform can be used in a magnetic field to both isolate rare cancer-specific immune cells for diagnosis and then activate them for therapeutic purposes. Our lab focuses on engineering aAPC properties to improve cell enrichment and activation. One characteristic is aAPC size, which can help us determine geometric features important for T cell activation, enable the development of high throughput antigen-specific T cell identification, and enable more efficient rare cell recoveries. Additionally, we modify the type of signals provided by the aAPCs to study effects of different co-stimulatory molecules on T cell activation. Because these are magnetic particles they can be clustered to learn about the spatial requirements of T cell stimulating molecules.
By controlling biomaterial properties, we can improve targeting of specific immune cells, mimic how the body activates immune cells, understand more about T cell biology, and increase the efficacy of T cell therapies. To mimic APCs we can create artificial APCs (aAPCs) by coating activating signals to the surface of magnetic nanoparticles. This magnetic platform can be used in a magnetic field to both isolate rare cancer-specific immune cells for diagnosis and then activate them for therapeutic purposes. Our lab focuses on engineering aAPC properties to improve cell enrichment and activation. One characteristic is aAPC size, which can help us determine geometric features important for T cell activation, enable the development of high throughput antigen-specific T cell identification, and enable more efficient rare cell recoveries. Additionally, we modify the type of signals provided by the aAPCs to study effects of different co-stimulatory molecules on T cell activation. Because these are magnetic particles they can be clustered to learn about the spatial requirements of T cell stimulating molecules.
Engineering Artificial Lymph Nodes

Adoptive T cell therapy has shown great promise in mediating tumor regression, yet it is an extremely costly and time-consuming process to isolate patients’ cells, expand them to large enough numbers and reinject into patients. Often these cells can become exhausted due to such high levels of stimulation and being in an environment not similar to the body. Our lab takes inspiration from the tissue and stem cell engineering fields which have shown that ex vivo culturing conditions influence cell phenotype and activity and aims to engineer more in vivo like environments for T cell culture ex vivo. These microenvironments are termed artificial lymph nodes. We further aim to engineer scaffold based therapies to directly modulate T cell activity in vivo.
See more here
Analysis of Neo-Antigen CD8 T Cells
Neo-antigens, tumor epitopes that arise from mutations in cancer cells, are thought to elicit a more potent and less toxic T cell than tumor associated antigens (TAAs). Using nanoparticle based simulations, created by our lab, we can expand the rare tumor specific T cell responses to measure TAAs and neo-antigen specific T cell responses. Current work has identified neo-antigens through T cell expansion assays, and neo-antigens show an increased expansion ability compared to tumor-associated antigens and demonstrate the resistance of neo-antigens to tumor suppression. Future work will include the phenotype of the T cell populations and the effect of immunotherapies on the self and neo-antigen T cell populations.
