The James Laboratory is located in the Department of Pathology in the Johns Hopkins University School of Medicine. Our research focus lies at the intersection of skeletal pathophysiology and stem cell biology. Current subjects of study include bone repair and regeneration, osteoprogenitor cell characterization and use, and neoplastic bone. A partial description of current interests is presented below:
Neural influence on bone formation and repair
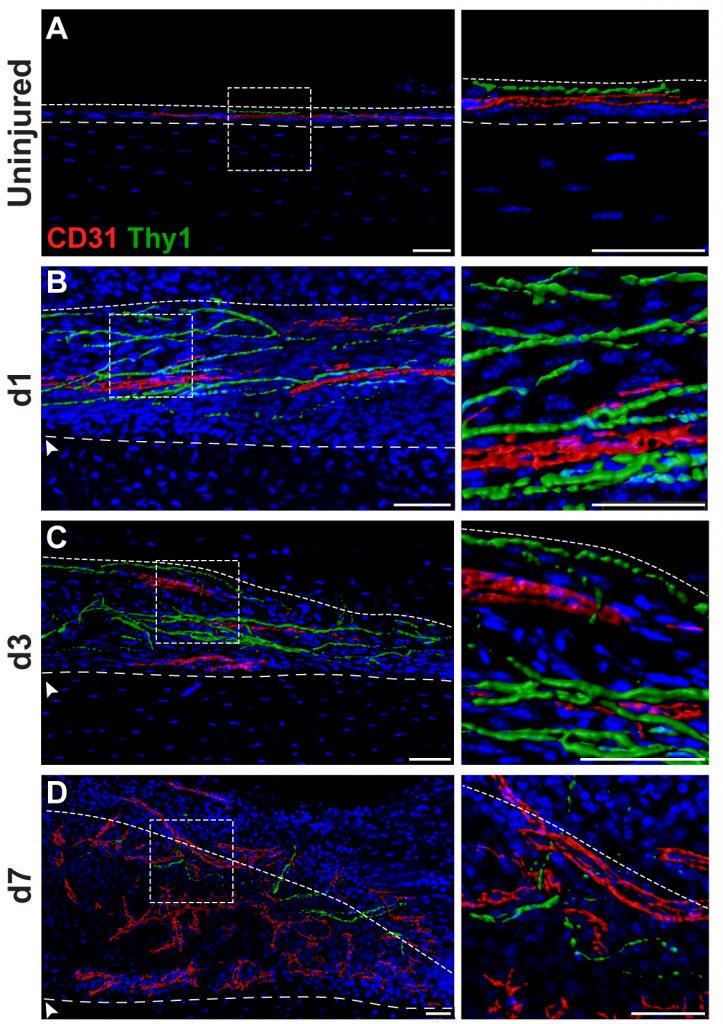
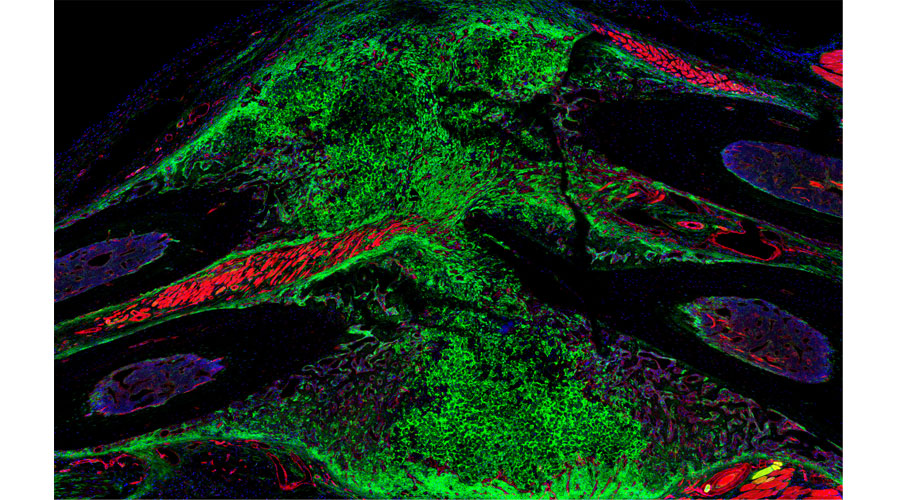
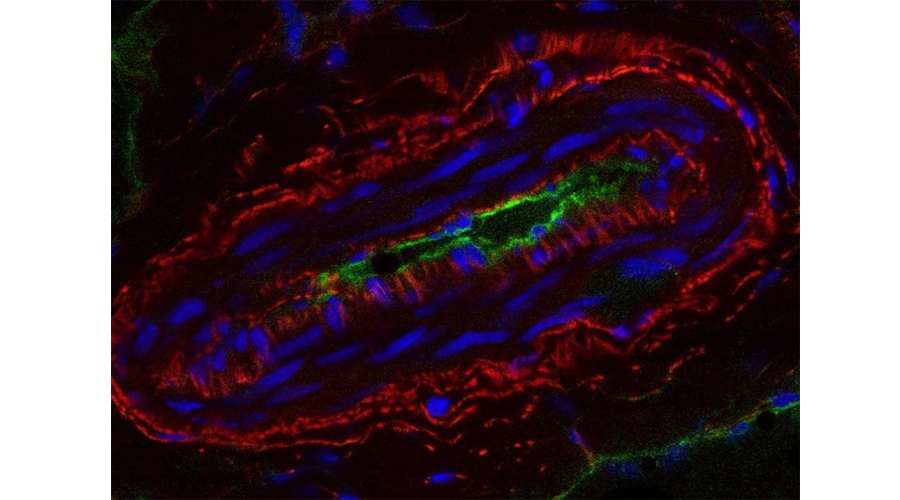
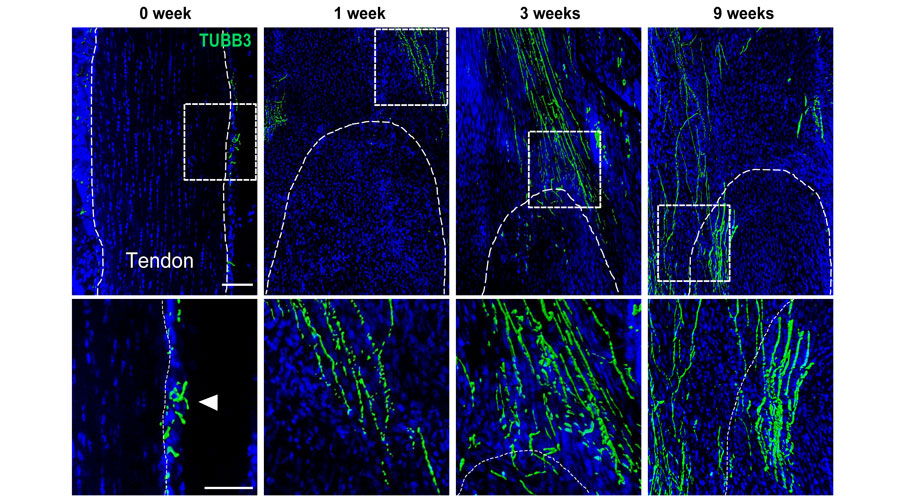
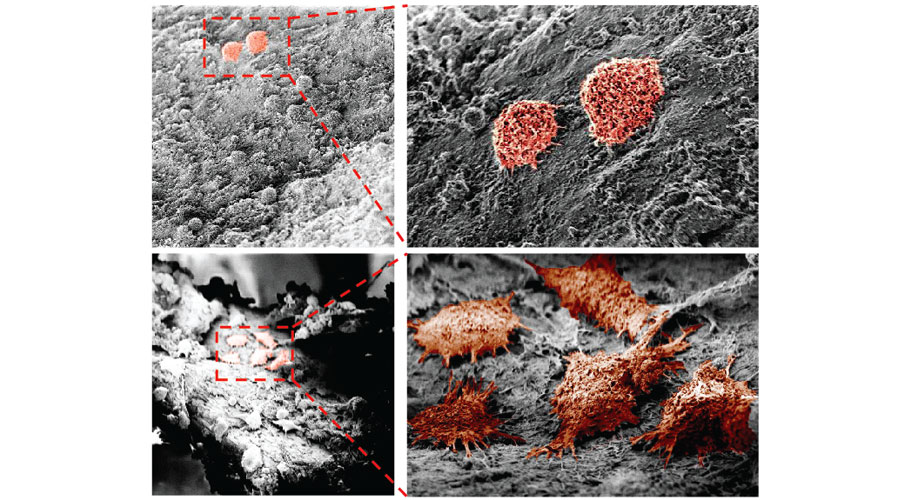
Skeletal sensory nerves are abundant in mature bone tissue, but their role in mammalian bone morphogenesis and repair is poorly understood. Our group studies the role of skeletal sensory nerves, focusing on understanding the crosstalk between peripheral nerves and bone-forming cells during tissue repair. Recently we have shown that NGF (Nerve growth factor) responsive TrkA (Tropomyosin receptor kinase A)-expressing sensory nerve fibers are integral for stress fracture healing in the mouse skeleton.
- A Neurotrophic Mechanism Directs Sensory Nerve Transit in Cranial Bone
- Fracture repair requires TrkA signaling by skeletal sensory nerves.
- NGF-TrkA signaling dictates neural ingrowth and aberrant osteochondral differentiation after soft tissue trauma
- Spatial transcriptomics reveals a role for sensory nerves in preserving cranial suture patency through modulation of BMP/TGF-β signaling
Perivascular progenitor cells
Mesenchymal progenitor cells reside in perivascular location across organs. Our past work has examined perivascular progenitor cells from human and mouse sources, their cellular characteristics and osteoprogenitor cell attributes. Present work focuses on the cellular and developmental hierarchy within this microanatomical stem cell niche. See below for several links to publications.
- Comparison of skeletal and soft tissue pericytes identifies CXCR4 + bone forming mural cells in human tissues.
- Human perivascular stem cell-derived extracellular vesicles mediate bone repair.
- Human perivascular stem cells prevent bone graft resorption in osteoporotic contexts by inhibiting osteoclast formation
- Lysosomal protein surface expression discriminates fat- from bone-forming human mesenchymal precursor cells
- Relative contributions of adipose-resident CD146+ pericytes and CD34+ adventitial progenitor cells in bone tissue engineering.
Novel differentiation factors for bone repair
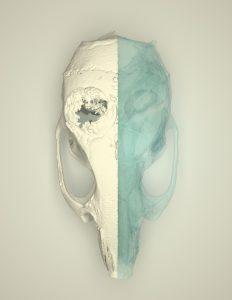
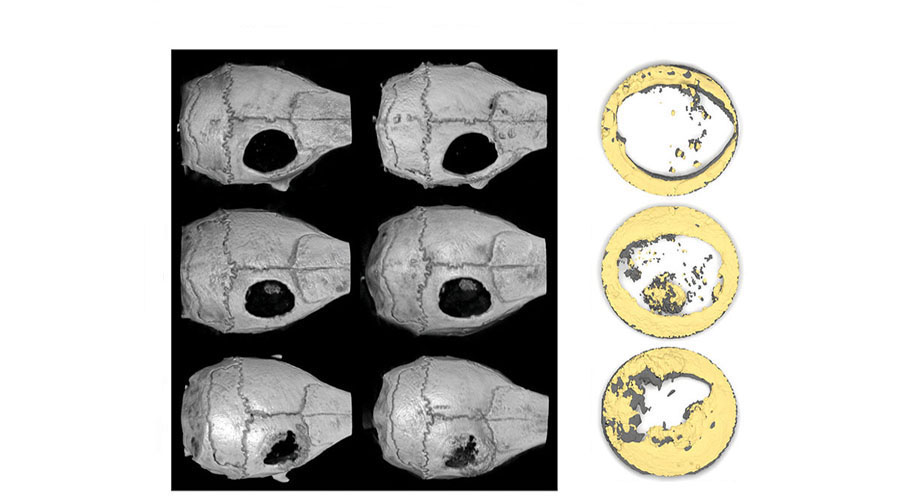
Numerous growth and differentiation factors are produced or released from bone after injury, some of which aid in the reparative process. We have shown that exogenous application of positive regulators of Hedgehog and Wnt signaling induce bone healing in both calvarial, axial, and appendicular skeleton. See below for several links to publications.
- Anti-DKK1 Enhances the Early Osteogenic Differentiation of Human Adipose-Derived Stem/Stromal Cells
- Calvarial Defect Healing Induced by Small Molecule Smoothened Agonist
- NELL-1 in the treatment of osteoporotic bone loss
- Combining Smoothened Agonist (SAG) and NEL-like protein-1 (NELL-1) Enhances Bone Healing
- WISP-1 drives bone formation at the expense of fat formation in human perivascular stem cells
Bone and soft tissue tumors
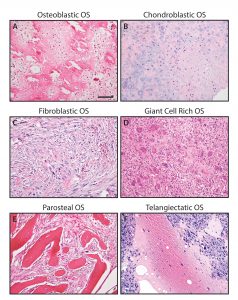
Malignant tumors of mesenchymal origin are a complex and heterogeneous group of aggressive tumors, termed sarcomas. Our group is most interested in skeletal sarcomas (osteosarcoma and chondrosarcoma), and perivascular soft tissue tumors. See below for several links to publications.
- Vascular patterning in human heterotopic ossification
- Sclerostin expression in skeletal sarcomas
- NELL-1 expression in benign and malignant bone tumors
- Pericytic mimicry in well-differentiated liposarcoma/atypical lipomatous tumor
- The pericyte antigen RGS5 in perivascular soft tissue tumors
- Ang-2 but not Ang-1 expression in perivascular soft tissue tumors